Anomalies
In case a study on DeepUnity PACSonWEB deviates from the corresponding study on the local PACS system, an anomaly will be registered. In this case, the anomaly is clearly indicated within the study page (DeepUnity PACSonWEB study page).
When opening the study administration pages for a study that has an anomaly registered, the anomalies tab will be displayed automatically and the label will be marked in orange.

|
Warning: The data of each listed anomaly are the data at the moment the anomaly was registered.
If patient or study data have been modified after the registration of the anomaly, these modifications will NOT be taken into account in the anomaly.
|
Anomalies on study level
The following anomalies will be reported:
• national number in DICOM files differs from HL7 information
• date of birth in DICOM images differs from HL7 information
• no or incorrect date of birth registered
• number of images in DeepUnity PACSonWEB differs from the number of images in the local PACS (taking into account any applied filtering)
• national number already registered with another patient through DICOM
• image or HL7 message received with the same study instance EUID or accession number but with other patient ID
• study for patient without ID is changed to a patient with ID via DICOM or HL7
• incompatibility with CvKO. (scroll to bottom of the page for more explanation)
• studies of different patients have been merged
• failed move or delete operation of image or series
For each anomaly, all relevant information is displayed in the overview.
From the overview, the related exam can be opened by clicking the study link.
Anomalies on patient level
The following anomalies will be reported:
• Multiple internal patient IDs have been registered for a single patient
• Received national number already linked to another patient
• Date of birth differs from date of birth that was registered with a previous exam
• National number already registered with another patient through HL7
• Patient without ID from DICOM (scroll to bottom of the page for more explanation)
• Patient without name from DICOM: a patient name is generated ("UNK_"+ [source abbreviation]+unique number) and used (scroll to bottom of the page for more explanation)
For each registered anomaly, the following information is shown:
• date and time when the anomaly was registered
• the anomaly type + the required access level of a user to be able to open the study
• a description of the anomaly
• the possible corrective measures
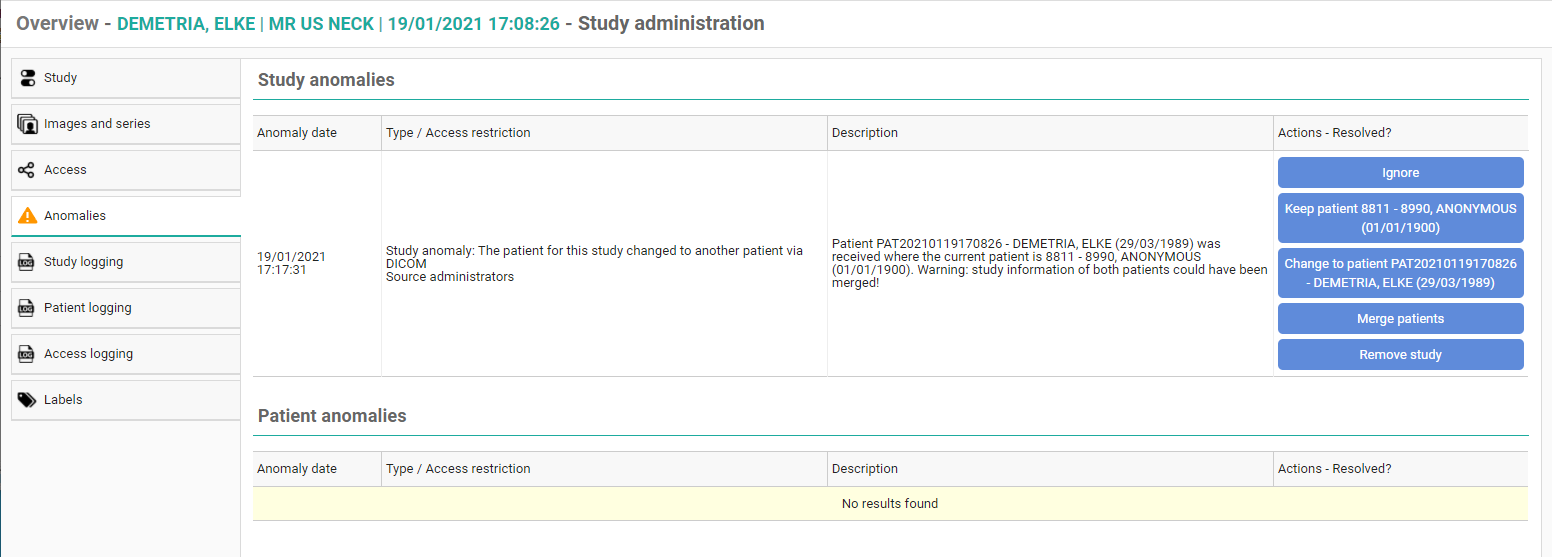
In case a national number was registered for two different patients, it will be possible to assign this to the first patient, the second patient or to merge both patients.
For the latter option, the following screen is displayed to perform the merge in a controlled way:
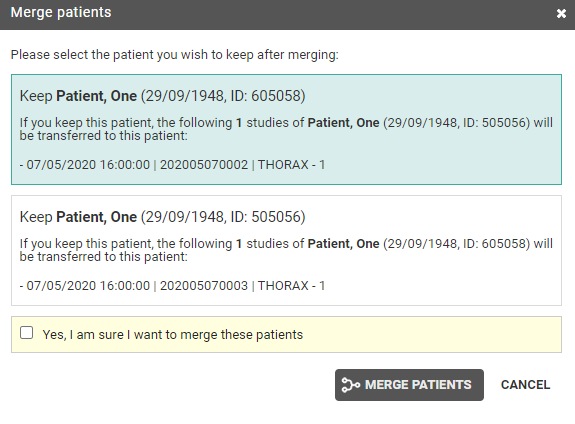
Once a solution has been selected, it will be marked in green. When hovering the cursor over the label, it is displayed by whom and when the selected solution was applied.
In case all registered anomalies have been marked as solved, the notification will no longer be displayed on the study page and the label 'Anomalies' on the study administration pages will no longer be marked in orange.
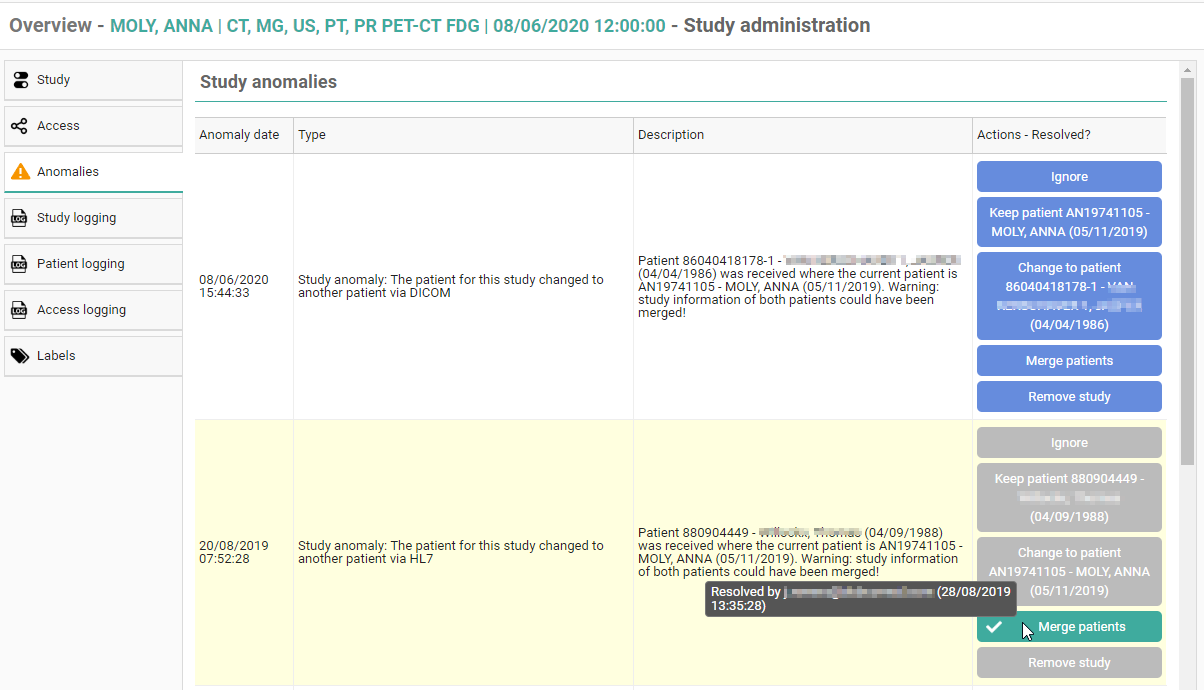
Study anomaly for patient without ID or name from DICOM
After fixing the study click on "Ignore" in the action description.
Only source admins can access the studies with these anomalies.
Study anomaly for incompatibility with CvKO
When DICOM files are received with DICOM tags that do no meet the requirements, 2 possible actions are possible to fix the anomaly.
Possible actions:
• Ignore: result is that nothing will happen and the anomaly will be resolved.
• Mark as corrected: a dialog is shown to send the corrected images to DU POW and share the study manually with CvKO.
All modifications with regard to anomalies on study level are registered in 'Study Logging' (Study logging).
All modifications with regard to anomalies on patient level are registered in 'Patient Logging' (Patient logging).